How to choose battery for your Electric Car: Things to Consider
Cell selection is one of the most important tasks for an electric FSAE car as the battery pack essentially fuels the car. This decision, made very early in the design stage affects not only the tractive system but also the performance of the whole car. This selection process is not an easy one to make as cells come in many different chemistries and various packaging styles; each with their own advantages and disadvantages. Ideally, you’d want to build a tractive system with excellent safety, high specific energy and specific power, good temperature characteristics, long cycle life and at low cost[1].
Among those many different chemistries that are used to construct a cell, lithium ion is the most common one, thus widely regarded as lithium ion cell. A lithium ion cell is an electrochemical device that can store and release energy. Lithium is the lightest metal
available in the world and it has become the replacement for lead in cell chemistries because of its lightweight
properties[1]. These days lithium ion cells find application in almost all electronic devices and even hybrid
and electric cars. The reason for this is very simple;they offer high energy density which means the devices
can be powered for longer. However, there are many types of Lithium batteries that need to be aware of and the challenge is to choose one that gives higher benefits for the user application.
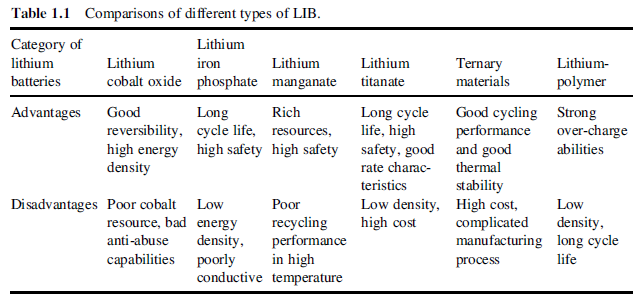
- Lithium cobalt oxide is most commonly used in handheld electronics and offers generally higher energy density and long cycle life although it is expensive, suffers from being less stable at higher temperatures and more reactive than other chemistries. This means that at about 130 °C the cell will enter the thermal runaway stage which is much lower than other lithium-ion chemistries.[2]
- Lithium manganese nickel cobalt (LiMnNiCo) shows a relatively high nominal voltage of about 3.6–3.8 V per cell and has one of the highest energy densities in a production cell today. [2] These cells can have either a high specific energy or high specific power but not both [3] and since we require a combination of both for our race car. This is not a suitable choice.
- Lithium manganate offers high energy and high power, however, suffers from shorter cycle life thus making it an appropriate chemistry to be used in portable power applications where you want long run time but not necessarily in automotive applications where you want long life.[2]
- Lithium iron phosphate offers high usable energy and is very abuse tolerant. These cells have a nominal voltage of 3.3V and an operating voltage range between 2.0V and 3.6V. This is lower than other chemistries such as lithium manganate (4.2V) or lithium polymer (3.7V, 4.2V). The lower voltage of Lithium iron phos- phate means that more cells are needed in series to achieve a given system voltage, and the watt-hour content is correspondingly lower for a given amp-hour capacity. [2]
While safety will always be paramount, it will be interesting to see what cells will be used in the future to maximise performance, reduce weight and enable easy cell replacement in a battery pack.
[1] Mehul Oswal, J. P. (2010). A comparative study of Lithium-Ion batteries. University of Southern California.
[2] J. Warner, The handbook of lithium-ion battery pack design: chemistry, components, types and termi-
[2] J. Warner, The handbook of lithium-ion battery pack design: chemistry, components, types and termi-
nology. Elsevier, 2015.
[3] “Lfp or nmc battery?” May 2016. [Online]. Available: https://www.betterworldsolutions.eu/ lfp-or-nmc-battery/
[3] “Lfp or nmc battery?” May 2016. [Online]. Available: https://www.betterworldsolutions.eu/ lfp-or-nmc-battery/
Comments
Post a Comment